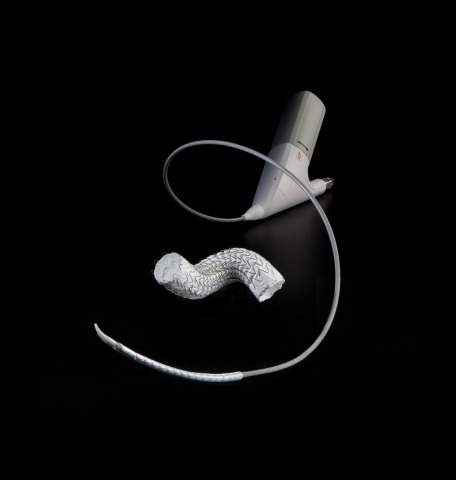
The reduced profile allows physicians to perform TEVAR in patients with smaller vessels where access is challenging and aortic anatomy is tortuous, expanding the availability of Gore’s thoracic stent graft to a greater population of patients.
The GORE® TAG® Conformable Thoracic Stent Graft was modified to reduce the delivery profile of the 31, 37, and 40 mm device diameters by 2 Fr for all lengths. The reduction in profile, through the use of a smaller diameter primary sleeve, is the only change to the device.
The stent graft leverages the same predictable outcomes of the time-tested Conformable GORE TAG Device, which has established long-term freedom from reintervention (93.1%), low complication rates, with zero migrations, fractures, or compressions. and a low 2.4% access site complication rate for endovascular treatment of the descending thoracic aorta.
The new profiles also continue to feature the same unique combination of proprietary ePTFE graft material and a fully supported, nested, nitinol stent.
“As the market for TEVAR has continued to expand and evolve, lower profile devices have rapidly emerged with a goal of increased patient applicability, accessibility, trackability and finally reducing access complications for patients with smaller vessels,” said Professor Boeckler.
“It’s reassuring that the new reduced profile sizes of the GORE® TAG® Conformable Thoracic Stent Graft was achieved without changes to the stent graft. Gore’s device is known for its conformability, and the GORE ACTIVE CONTROL System enables me to take full advantage of the conformability by allowing precise placement during TEVAR procedures. The combination of controlled delivery with the trusted stent graft, and now reduced profile for the device, is a significant advancement and approaches unmet needs performing TEVAR in my daily practice.”
The new reduced profile devices continue to feature the GORE® ACTIVE CONTROL System, which facilitates optimized wall apposition even in complex anatomies, such as acute aortic angles. Now, the additional reduced profiles on critical sizes needed allow the device to be introduced through a smaller sheath.
“This milestone is one of several that illustrate our continued dedication to innovate our devices for better long-term patient care,” said Erik Davies, vascular global marketing leader at Gore. “We are proud that we were able to reduce the profile of the GORE® TAG® Conformable Thoracic Stent Graft without changing the design of the device. Patients with smaller vessels are now able to receive the same conformable device that has demonstrated trusted and safe performance for the past 20 years. Maintaining the integrity of the device, Gore’s launch of reduced profiles on critical sizes showcases our continued commitment to developing solutions that advance endovascular solutions for diseases of the aorta.”
The GORE TAG Conformable Stent Graft with ACTIVE CONTROL System is part of the growing family of endovascular products that share a mission to effectively treat aortic disease, backed by Gore’s highly rated clinical support team and educational offerings.
The portfolio of products includes the GORE® EXCLUDER AAA Endoprosthesis for the treatment of abdominal aortic aneurysms (AAA), as well as the GORE EXCLUDER Conformable AAA Endoprosthesis with ACTIVE CONTROL System, also approved in EU. Together, the GORE EXCLUDER device family is indicated to treat the broadest range of abdominal aortic aneurysms in patients with challenging anatomies.
The GORE EXCLUDER Iliac Branch Endoprosthesis is approved in EU and is the first FDA-approved, off-the-shelf device for the endovascular treatment of common iliac artery aneurysms or aortoiliac aneurysms, and is indicated for use with the GORE EXCLUDER AAA Endoprosthesis. For potential additions to its branched portfolio, Gore continues to conduct studies on new and novel technologies to treat other aortic disease states.
Source: Company Press Release






