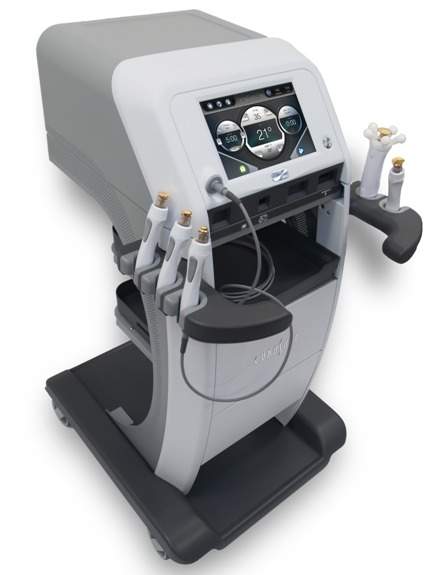
According to Hologic, the new TempSure RF platform enhances the ability of clinicians in performing both surgical and non-surgical aesthetic procedures across a variety of specialties, with the help of a single device.
TempSure Surgical RF technology uses a 300-watt and 4-MHz radiofrequency platform, which is said to offer precise incisions with minimal lateral thermal damage to the surrounding tissues.
“The resulting coagulation lessens sparking and charring during procedures and quicker recovery and better healing in patients can be seen,” Hologic said in a statement.
The device, which enhances patient satisfaction and aesthetic outcomes, can be used by clinicians in a wide range of specialties such as plastic surgery, dermatology, gynecology and ophthalmology.
TempSure Surgical RF has been designed to enhance the existing TempSure radio radio-frequency platform. It has a variety of electrodes that integrate with the main TempSure unit.
In January this year, the company rolled out TempSure Envi, a device to treat facial fine lines and wrinkles, tightening the skin through soft tissue coagulation and to temporarily reduce the appearance of cellulite.
Cynosure has also re-launched TempSure Vitalia hand pieces and probes to the market. It will continue to sell its MonaLisa Touch CO2 laser after the US Food and Drug Administration (FDA) inquiry on products used in energy-based women’s health procedures.
Hologic said that Cynosure worked closely with the FDA and reviewed and updated all its marketing and promotional materials to ensure they are in line with the labeling expectations of the FDA.
Hologic’s Cynosure division president Kevin Thornal said: “We’re continually innovating to ensure our customers set themselves apart with effective and diverse treatment offerings, and this enhanced platform is a gamechanger across specialties.
“The cutting-edge technology of the TempSure platform will now allow doctors to transition seamlessly from invasive to non-invasive treatments on one device.”






