Roche will develop companion diagnostic for use with Keytruda (pembrolizumab), Merck’s anti-PD-1 therapy, in advanced solid tumors with mismatch repair deficiency (dMMR).
Roche tissue diagnostics head Jill German said: “We are excited to collaborate with Merck to develop a pan-cancer companion diagnostic test panel to detect mismatch repair deficiency.
“This new development could help change the way we identify patients best suited for immunotherapy treatment.”
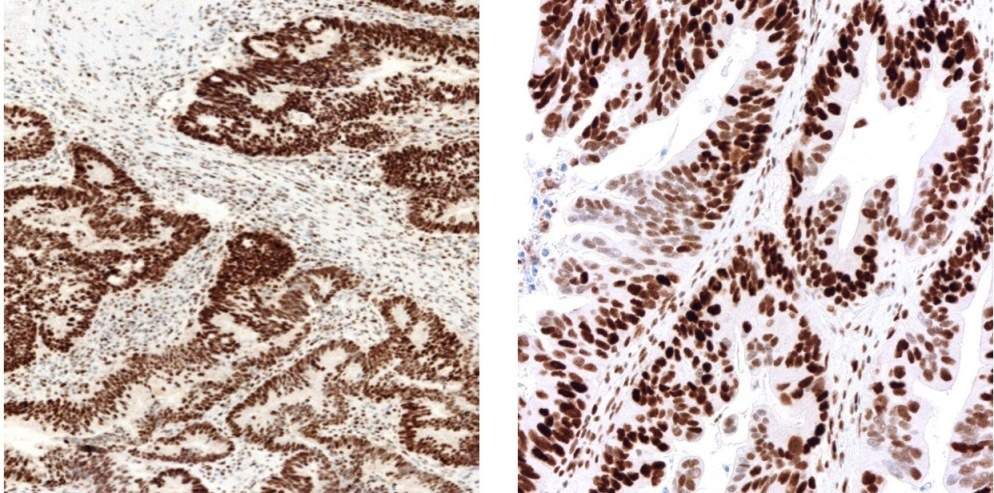
Roche said The development of MMR immunohistochemistry (IHC) assay is expected to pave the way for taking treatment decisions, based on biomarker expression within solid tumors originating from various parts of the body.
Merck Research Laboratories oncology clinical development senior vice president Eric Rubin said: “A key element of our strategy at Merck is focused on identifying those patients likely to benefit most from our medicines.
“We look forward to working with Roche to develop a diagnostic test for mismatch repair deficiency.”
Last year, Merck’s anti-PD-1 therapy Keytruda had become the first cancer treatment approved by the the US Food and Drug Administration to treat adult and pediatric patients with unresectable or metastatic, microsatellite instability-high or mismatch repair deficient solid tumors that have progressed after prior treatment and who have no other alternative treatment options, or colorectal cancer that has progressed following treatment with certain chemotherapy drugs.
Testing for MMR includes immunohistochemical detection of four MMR proteins (MLH1, MSH2, MSH6 and PMS2). These arrays along with BRAF V600E, are presently being used to help in identifying genetic predisposition for colorectal and other cancers called Lynch syndrome.
The collaboration between Roche and Merck is expected to expand the utility of this panel to include selection of patients with solid tumors for immunotherapy.
As per the Swiss healthcare company, the companion diagnostic which is being developed by IHC for used on Roche BenchMark ULTRA instrument, is claimed to be a widely installed IHC/ISH (in situ hybridization) staining platform globally and can offer broad testing access to patients.






