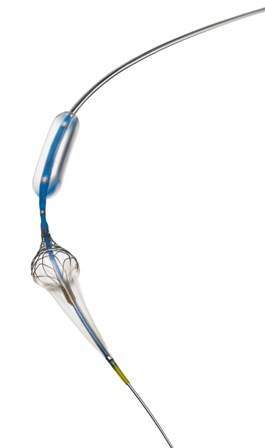
Claimed to be the first of its kind, Paladin is provided with an angioplasty balloon and integrated 40-micron filter.
The micro-embolic protection offered by the Paladin filter will serve as an added measure of protection, and provides procedural flexibility in carotid stent procedures.
Paladin device enables to adjust the size of the embolic protection filter in vivo to suit each individual patient’s anatomy.
The device, which already secured CE mark, has been used in more than 1,400 patients in Europe.
Contego assessed the Paladin device in 106 registry patients undergoing carotid artery stenting with various stents, including open cell, closed cell, and mesh covered designs.
Paladin system with IEP is indicated for PTA in the carotid arteries with capture and removal of embolic material.
The device can also be used for post-dilation of self-expanding stents in the carotid arteries with capture and removal of embolic material.
According to the company, the diameter of the arterial site for filter deployment should be less than 7.0mm, and Paladin system with IEP must be used along with an available embolic protection device.
Contego Medical founder and CEO Dr Ravish Sachar said: “We are thrilled to have achieved this milestone for the first of several devices Contego Medical intends to bring to the U.S. market.
“The Paladin System utilizes our patented Integrated Embolic Protection (IEP) technology, aimed at providing superior performance while improving the safety profile of existing cardiovascular procedures.
“With the strength of our clinical data and the unique promise of our IEP technology, we look forward to positively impacting patient care and outcomes.”
Contego Medical is engaged in the development and commercialization of next-generation medical devices for the treatment of neurovascular, coronary and peripheral vascular diseases.
The firm’s IEP platform aggregates embolic protection and treatment into one device, enabling to simplify catheter-based procedures and enhance patient outcomes.






