Infectious diseases bring misery to millions every year. A hard-hitting virus can rapidly sweep through populations and, in some cases whole continents, in a matter of weeks.
Effective treatment often relies on antiviral drugs being administered within the first three days of the onset of symptoms, so the value to medical staff of having easy-to-use, quick and reliable ways to test patients to hand, whether they are in a local hospital or clinic, a doctor’s surgery or an isolated village that is hundreds of miles from the nearest healthcare facility, cannot be overstated.
As a result, there is a strong international trend to move the testing and monitoring of infectious diseases outside of the confines of specialist hospitals by using point of care (PoC) diagnostics. Most recently, a generation of highly advanced testing and detection technologies have been developed using the advantages offered by new biosensor devices and smartphones.
The most advanced PoC diagnostic approach combines specialist biosensor materials and advanced electronics in handheld devices for the accurate detection of illnesses from patient-supplied samples.
SAW: the future
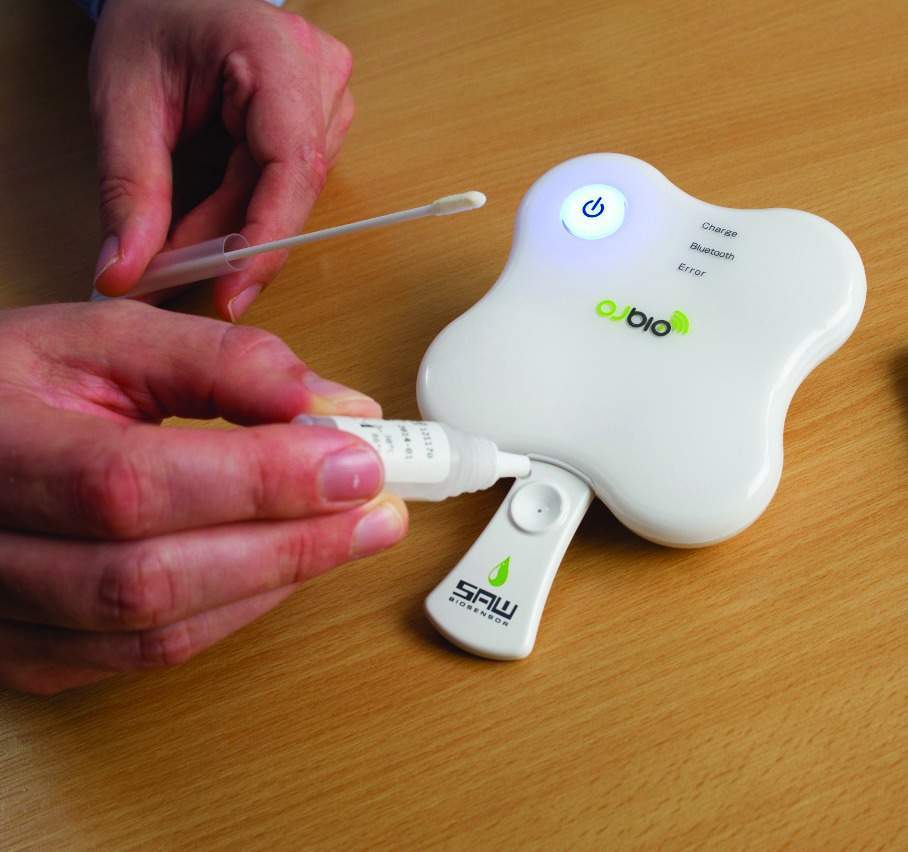
One example is a new smartphone-enabled biosensor from medical device and diagnostic company OJ-Bio, a joint venture between the Newcastle-based nano biotechnology company Orla Protein Technologies and the Japan Radio Company (JRC).
In this collaboration, Orla provides the specialist biocapture proteins that are combined with JRC’s advanced electronics capability to create a specialist multichannel biochip technology platform.
This work has involved taking shear horizontal surface acoustic wave (SAW) chips and coating them with new biocapture protein technology to create a device that gives highly specific responses when it comes into contact with samples containing markers of the diseases concerned.
SAW chips have been in commercial use for over 40 years and are widely deployed in the telecoms industry and in base stations where they act as bandpass filters. Now, for the first time, the combination of novel antibodies, nanoparticles and multiplexing methods enables SAW technology to be used to its full potential with biological material.
When samples, from serum, urine, saliva or blood, are applied to the biochip (held in a disposable cartridge), the presence of a disease antigen causes a shift in the phase angle of the surface acoustic wave passing across the chip surface and this is translated into an electronic signal. The signal can be detected and converted into a ‘yes’ or ‘no’ result for the presence of a disease.
The new chips are designed and manufactured to allow operation in liquids with high sensitivity to perform as an immunoassay device that detects the mass and viscosity changes of a biological molecule on the surface of the SAW sensor devices. This also enables the system to provide an accurate measure of the target biomarker present, as well as a simple positive or negative outcome.
The sensor area is modified with capture antibodies that are specific to each target marker and are held in the correct orientation to react with disease antigens. When the target marker in the solution binds to the immobilised capture antibody, the binding causes a shift in the phase angle of the surface acoustic wave passing across the chip surface, producing an electronic signal.
Different capture coatings can be tested immediately on a working prototype measurement unit so that robust assays can be developed.
In this way, disposable SAW sensors can be inserted into an electric reader to detect multiple antigen-antibody reactions via changes in the phase/amplitude of the input/output signals.
Speedy results
Importantly, the diagnostic device can be used at a patient’s bedside, or other point of care location, such as a GP surgery, health centre or pharmacy, with the results being available immediately, without requiring laboratory analysis. This capability also enables the device to be used in the patient’s home.
The results from the biosensor device can be read on an app-enabled, handheld device, such as a smartphone, and the data can also be wirelessly transmitted to a central resource for remote connectivity to healthcare networks, records and facilities.
This capability therefore makes the technology ideal for remote disease monitoring, with many applications for rural use or situations where clinical readings are needed without patient transfer.
As part of initial research into the development of accurate, rapid and low-cost, point of care for respiratory viruses, the SAW biosensor has been developed and tested in collaboration with the Health Protection Agency (HPA) in Newcastle (now Public Health England) for the detection of a number of respiratory viruses.
This initial work was carried out into the detection of three respiratory viruses, influenza A and B viruses, common flu strains previously linked to some major epidemics, and respiratory syncytial virus (RSV), a major cause of coughs and chest infections.
The data obtained confirmed the ability of the technology to provide accurate results in around ten minutes, had good levels of diagnostic sensitivity for the three test viruses (total 93% when compared with the HPA lab-based PCR method, the ‘gold standard’ for respiratory testing) and did not give false positives, showing 100% specificity, even when other viral analytes were present.
As such, the device effectively demonstrated the potential for a low-cost tester that could revolutionise the speed of diagnosis and treatment, hitting the flu virus hard at its source and inhibiting its ability to spread.
Building on the results of this initial test work, a new prototype handheld device has been developed that incorporates a multichannel biochip. Formal clinical trials using the product to detect the three respiratory viruses mentioned above are due to start in 2015.
However, because the platform biosensor technology allows the detection of different protein biomarkers, the potential application areas for the new device are very broad. For example, considerable work has already been undertaken on the use of the specialist biochips for the detection of periodontal gum disease, and applications have been identified for animal testing application in the veterinary sector.
CRP and easy
Another area of interest is the capability of the device to help in reducing problems associated with the overprescribing of antibiotics. Here, as well as providing a fast and accurate diagnosis for flu that could help a GP demonstrate, and explain, why antibiotics may or may not be appropriate, the device can also be used to detect the presence of C-reactive protein.
CRP is a recognised biomarker of inflammatory disease, which can be used to rule out serious bacterial infections. Although the CRP test is not new, its availability on a PoC diagnosis device, potentially for use by patients themselves as well as frontline healthcare staff, could provide a useful tool in the reduction of the inappropriate prescribing of antibiotics for flu-related conditions.
In other areas of other infectious disease identification and control, OJ-Bio is also working with researchers from University College London (UCL) to develop a device capable of detecting HIV marker proteins in human blood quickly and effectively.
So far, the technology has been proven to work using model HIV samples, where the inherent sensitivity to low levels of multiple markers has the potential for much earlier diagnosis of the disease. Eventually, it is hoped that the technology will empower patients to gain earlier access to antiretroviral treatment with better associated health outcomes.
More broadly, effective testing and diagnosis using portable devices could have potential applications in community screening programmes, although there will always be a need for definitive, laboratory-based molecular testing methods capable of screening for all types of infectious agents.
However, the ability of PoC testing to deliver a fast and accurate result will have significant benefits in certain situations, and particularly those in which quick diagnosis is essential. For example, if a patient with a suspected condition arrives at a healthcare facility during ‘out of hours’ periods, the PoC device would enable an immediate diagnosis to be made, allowing clinical decisions to occur quickly, with laboratory verification or confirmation testing being available later.
PoC testing using innovative medical devices is now one of the fastest growing fields of laboratory medicine and is becoming increasingly prominent on the international healthcare agenda. As a result of this work, new biosensor technology using multichannel biochips has the potential to provide an effective solution to modern-day infectious disease diagnosis and control challenges.






