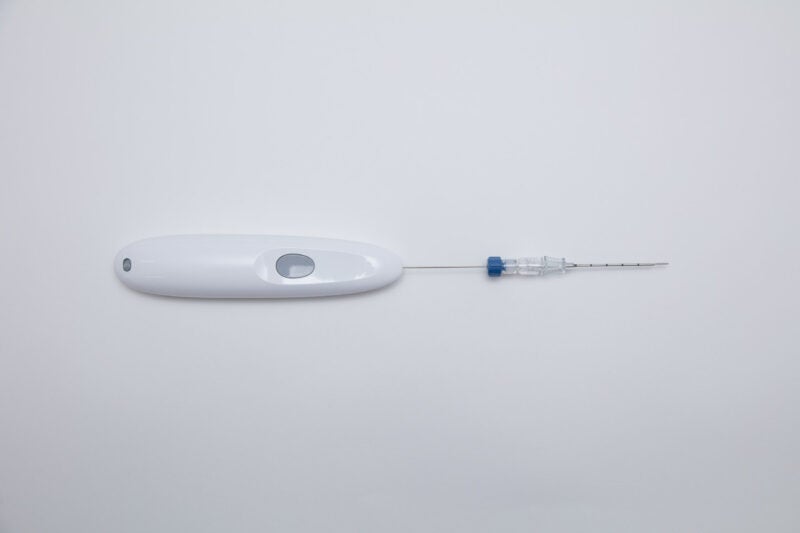
Single Pass has signed an exclusive distribution agreement with Mermaid Medical Group to distribute the former’s biopsy closure device to healthcare professionals in the US market.
The Single Pass biopsy closure device is an advanced device intended to improve the results of biopsy closure procedures and patient care.
The device is said to offer a safe and effective way to close biopsy sites, lower the risk of complications, and speed up patient recovery using its latest technology and user-friendly design.
Under the distribution deal, Mermaid Medical Group will act as the sole distributor of the biopsy closure device in the US.
Mermaid Medical Group, a medical technology company, will use its broad network and expertise in the medical technology sector to distribute the products.
The firm will make widespread availability of the biopsy closure device and ensure efficient delivery of this device to healthcare facilities across the US.
Single Pass CEO Bill Colone said: “We are thrilled to partner with Mermaid Medical Group for the exclusive distribution of our biopsy closure device in the United States.
“Mermaid Medical Group’s reputation for excellence and their commitment to advancing patient care aligns perfectly with our mission. Together, we aim to revolutionise biopsy closure procedures and improve patient outcomes.”
Single Pass said the collaboration between both firms represents a noteworthy achievement in the field of biopsy closure technology.
The integration of Single Pass’s device with Mermaid Medical Group’s broad distribution network is expected to provide US healthcare practitioners with an enhanced biopsy closure technique solution.
Mermaid Medical Group general manager Marcus Nielsen said: “We are excited to collaborate with Single Pass and introduce their groundbreaking biopsy closure device to the US market.
“This partnership reflects our dedication to providing healthcare professionals with innovative solutions that enhance patient care.
“We look forward to working closely with Single Pass to make this device widely accessible to medical facilities across the country.”






