
Conductive ink sounds like a misnomer. Ink is used in our pens and printers, not as a vehicle for current in electric circuits, right? Wrong. Membrane keyboards, solar panels and credit cards are just three examples where we might find conductive inks at work. Comprised of conductive organic polymers in solvents, metallic particles suspended in binders or organic-metallic blends, they’re typically 3D printed onto a substrate, where the solvent evaporates with the application of heat and a conductive pattern remains. A key advantage of printing electronics in this way is the ability to maintain the conductive properties of a device along a non-planar surface. It’s for this reason that one of the more recent applications of conductive ink is in medical wearables. Using a range of materials, including carbon nanotubes, graphene and gold nanowires, research teams have created devices that can tolerate a certain amount of flexing and stretching for the sake of patient comfort, as well as to ensure enough of a contact point with the skin to monitor a range of analytes detectable in sweat. The other big frontier for flexible electronics is continuous glucose monitoring; currently done using microneedles connected to microelectronic devices that collect and transmit the data, researchers now believe that same data can be extracted from interstitial fluid secreted by the skin, using flexible electronics.
Muscle and bone
Despite all this potential, the area that still eludes researchers working with flexible electronics is motion at the musculoskeletal level. It might not seem that important to measure at first glance, but doctors can draw conclusions about a range of diseases from patterns of contraction and relaxation in the muscles. The most obvious of these is Parkinson’s disease, characterised by rigid muscles and rhythmic tremors. The reason this function remains elusive, according to Dr Akhilesh Gaharwar, associate professor of biomedical engineering at Texas A&M University, comes down to one vital limitation.
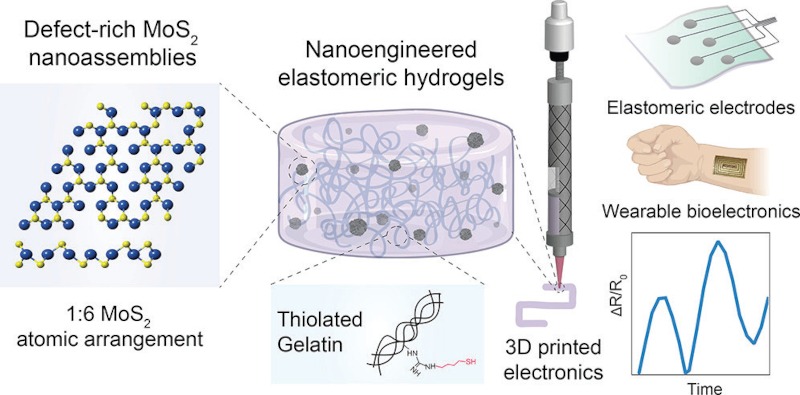
“All of these materials can provide electrical conductivity, but they cannot provide strain sensing,” he explains. “Our material is able to provide both. That’s what separates our bioink from other inks.” The discovery of the ink itself takes us back almost a decade, when Gaharwar started working on injectable hydrogels after prior research on material-cell interactions. “At the same time, additive manufacturing was becoming a hot field, so I decided I could use my hydrogel skills to make inks for 3D printing,” he says. “More recently, we asked ourselves whether we could impart some sort of unique property to these hydrogels.” Conductivity was the property of choice, but because hydrogels have similar properties to water – which in its purified form is a poor conductor of electricity – Gaharwar and his colleagues had to add another ingredient to the bioink: nanoparticles.
Not everything within this class of material is conductive, but integrating particles of metals on the nano scale is a common way to add conductivity to inks, with silver, gold and copper popular choices. But while these options work fine for inks used in consumer electronics, medical wearables must contend with the risk of cytotoxicity, as nanoparticles can pass through into the deep layers of the skin and cause toxic side effects. It’s for this reason that Gaharwar settled on 2D molybdenum disulfide. “In that particular nanomaterial you have two different atoms: molybdenum and sulphur,” he explains. “If you change the ratio between the two, you can create atomic vacancies, which make the material electrically conductive as well as highly reactive to bonding.”
Gaharwar and his colleagues then modified the ends of the polymers to make hydrogels with a sulphur bond. “When we mixed that together, we observed that these polymers and nanoparticles clicked together to form a covalent bond, making a stronger gel and because MoS2 is electrically conductive, it also made the hydrogel electrically conductive,” he says.
Under the skin
The implications of this could be significant. For wearable medical technologies, the benefit of a stretchable, skin-like material that is also conductive could mean more comfort for patients and a better vehicle for sensors to detect analytes in sweat and interstitial fluid. But the high biocompatibility of Gaharwar’s bioink could also open avenues for an injectable electronic hydrogel with the ability to sense muscular motion. This is the new frontier that he and the Texas A&M researchers working with him wish to reach.

“We are trying to see if [the hydrogel] can be implanted inside the skin, and from there if it can sense the motion of tissues. It will get implanted into the skin and stick to the muscle, where it will get stretched and relaxed, and the changes in electrical conductivity will be sensed.”
Gaharwar frames the process as being a bit like getting a tattoo, just one that could aid in the research and detection of diseases with muscular contraction and relaxation as a symptom, like Parkinson’s. “It will be similar, but rather than a person drawing a design on the skin, it will be a machine,” he says. “For Parkinson’s, we’re trying to see whether there’s a pathological feature we can detect and by getting data directly from the movement of muscles, we can better analyse what exactly is happening to the body.”
Gaharwar and his team haven’t stopped at muscle sensing though, with ambitions to use the capabilities of their bioink to bridge the gap between human and robotic movement. “If we think a little further ahead, our tattoo can also act as an interface between an exoskeleton and the body,” he says. “It can sense the movement of a muscle and transport it to an exoskeletal device.” It’s not hard to imagine use cases where such a device could be life changing – volumetric muscle loss or muscle weakening due to nerve damage to name just two – but although the Texas A&M team have filed a patent on the bioink, Gaharwar admits there’s an element of future gazing to having it enable robotics. Along the path of researching the bioink, the Texas A&M team also published an open-source manual in the journal GEN Biotechnology with the express goal of democratising bioprinting among research teams. “If you want to go and do some bioprinting, a bioprinter will cost you ten thousand dollars,” he says. “To make the technology accessible to other people, we developed an open-source protocol to convert a normal 3D printer into a bioprinter.”
Bringing bioink to the people
In terms of an actual roadmap towards commercialising the bioink, like most exciting new technologies in the medical world, there are regulatory barriers that require testing it over the long term. Gaharwar says the team have already done some biocompatibility studies in animal models as part of their most recent paper on the bioink, but that they’re yet to evaluate how fast the hydrogel degrades, and whether this or any other time-related factor affects the performance of the ink in situ. These longer-term challenges, he says, are currently being tested through a collaborator using more animal models. The team currently have funding for the next “two or three years”, according to Gaharwar, but they’re attempting to extend their runway by applying for grants from the likes of the National Health Institute, which he also hopes will expand the scope of research to include Alzheimer’s disease, where he believes the biocompatibility and conductivity of the bioink could assist in the firing of neurons that have lost their ability to connect with each other, once it’s injected into the right location in the brain. Gaharwar and the Texas A&M researchers will continue to validate their bioink for a range of uses, the simplest of which might be improving medical wearables, but if the more ambitious goals requiring injecting it into the body offer hope to those with incurable diseases, the future-gazing will have been time well spent.






