
Brain-computer interfaces (BCI) or brain-machine interfaces (BMI) enable direct information transfer between the brain and a technical circuit. By reading thoughts – like those involved in mental commands – they can mediate machine control independent of speech and movement. Being that they do not require the actuation of a muscle, BCIs enable revolutionary control capabilities. However, their implementation is hampered by significant technological challenges, which explains the high development effort in this area. Recent studies have shown that physically impaired people currently benefit the most from the realisation of BCI/BMI, as they have the potential to enable patients with paraplegia and locked-in syndrome to communicate, write and eat again. Regular exercise with BCIs can even lead to the regeneration of motor skills. In addition, BCIs are used for brain research, such as brain mapping and studying neuronal diseases such as epilepsy and brain tumors.
BCI can be classified into non-invasive and invasive. Measurement of brain activity in invasive BCI proceeds via an electrocorticogram (ECoG) which is placed on the dura – one of the layers of connective tissue that make up the meninges of the brain – to measure spikes or local field potentials (LFPs). Implantation of these BCIs provides the highest resolution, making it of particular interest for brain research – but is also the most invasive method. Due to the implantation of microelectrodes directly at the dura and in the cerebral cortex, infections and bleeding can easily occur. As a result, this technique cannot be used permanently and is currently relegated to the laboratory. Also, a repeat surgery is required to remove the BCI, presenting further patient risk. Due to these limitations in use, there is currently no data on long-term stability.
The right signal
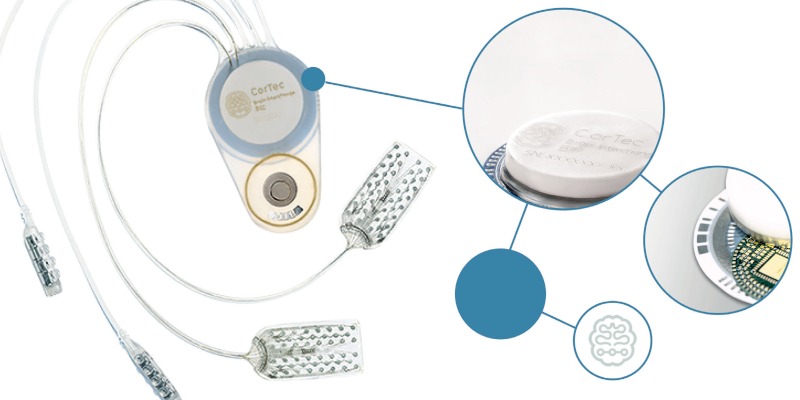
The medical technology company CorTec is working with clinical partners to produce an invasive medical device that is stable over the long term. The device would measure LFPs to convert neural signals into actions from external devices. With this, the neural signal can be transferred into an electrical signal to control a computer, prothesis or wheelchair. Here, the ‘data processing’ takes place completely outside the body using wireless electronics, meaning patients can benefit from new developments in data processing, like more powerful hardware.
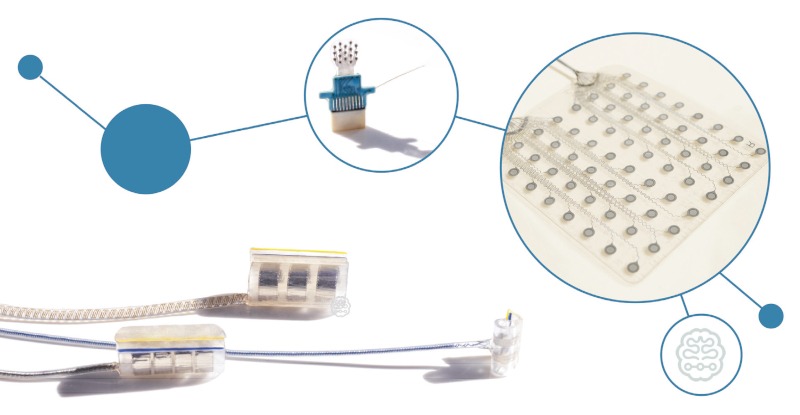
The implantable system is designed for bidirectional use, which means both the central and peripheral nervous systems can be recorded and stimulated. The next phase of research will further refine the technology, preparing it for initial first-in- human studies. Spinal cord injuries at or above the C8 cervical vertebrae carry a high risk that the corresponding patient will become a quadriplegic, preventing the use of arms, hands, and the lower extremities. The French company STATICE is researching to restore mobility by reading thoughts and linking them to an exoskeleton using invasive – but long-term functional – implants. The results of a clinical trial were published in the The Lancet at the end of 2019, providing proof of concept for the control of an exoskeleton for quadriplegics. The researchers used WIMAGINE neuroprostheses in the study. They consist of 64 electrodes and can be placed on the dura to record brain activity. The long-term functionality of the WIMAGINE implants was demonstrated by implantation in two patients with C5 spinal cord lesions. The next step will be to confirm this in additional patients. In addition, WIMAGINE is being used to bypass the damaged spinal cord so that the motor signals originating from the brain can be decoded and translated directly into impulses to the nerves. Thus, the damaged spinal cord can be bypassed wirelessly.
Going quantum
BCI systems can also be combined with non-invasive brain stimulation by means of an Electroencephalogram (EEG). Professor Soekadar’s research group at the Charite in Berlin is working on this. By combining these two technologies, neuroplastic processes can be better understood and influenced, allowing new and effective therapeutic methods to be developed. In this way, diseases of the CNS can be treated individually and with few side effects. The exoskeleton is controlled by means of an EEG and electrooculography. It has been shown that assistive and restorative BCI are effective clinical tools for regaining the ability to move. The major advantage of this non-invasive option is that it allows patients to remain mobile and leave the laboratory, but at the expense of resolution and frequency range. A possible further development of this system lies in the technology of quantum BCI, which uses quantum sensors. Due to a better contact between sensor and brain, a better sensitivity, selectivity and resolution can be achieved by using quantum sensors. In addition, the compactness can allow for better freedom of movement. The system should make it possible to control the complex movements of a robot or reconstruct language from neuromagnetic brain activity. Quantum sensors are thus the most promising technology to advance non-invasive BCI applications, but some technical challenges – magnetic shielding and thermal insulation among others – need to be overcome first.
Brain stimulation
Psychiatric disorders are another area of medicine where BCI devices are showing promise. Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation method used to treat several psychiatric disorders, including Parkinson’s disease and depressive disorders. Using TMS, a pulsed magnetic field is delivered via a coil to the targeted cortical region. An electric field is generated in the brain by electromagnetic induction. This induced electric field can then modulate neuronal activity non-invasively, greatly facilitating clinical application. However, TMS is unable to stimulate deep brain regions due to the rapid attenuation of the generated magnetic field, which distinguishes it from various conventional invasive brain stimulations. In contrast, temporal interference (TI) stimulation – also a non-invasive method – can stimulate deep brain regions. In a TI, several electric fields with high but slightly different frequencies are generated, which, due to the small frequency difference, form a temporal interfering field to which the neurons of the brain react.
With an increase in diseases with a neurological component – such as depression and chronic pain – the rising demand of minimally invasive surgery and an aging population more prone to brain diseases, research and development in the field of brain stimulation is increasing significantly. For instance, the global market size was estimated by Allied Market Research to be around $4.4bn in 2018 and is expected to reach $11.3bn by 2026, growing at a CAGR of 12.5%. This is divided into 24% Active Implantable Medical Devices, 35% Spinal Cord Stimulators, 21% Inner and Middle Ear Stimulators (Cochlea and BAHA), 14% Deep Brain Stimulators and just under 10% Sacral Nerve Stimulators. A glimpse of these and other exciting technologies was provided at the Compamed Innovation Forum 2022, held annually in the summer before the main COMPAMED conference.
“Recent studies have shown that physically impaired people currently benefit the most from the realisation of BCI/BMI, as they have the potential to enable patients with paraplegia and locked-in syndrome to communicate, write and eat again.”
BCI helps ALS patient communicate
Researchers at the Wyss Center for Bio and Neuro-engineering, in collaboration with the University of Tübingen in Germany, have enabled a man in his thirties with complete paralysis, who cannot speak, to communicate via an implanted brain-computer interface. The clinical case study has been ongoing for more than two years with the participant, who has advanced amyotrophic lateral sclerosis (ALS) – a progressive neurodegenerative disease in which people lose the ability to move and talk.
The participant, who lives at home with his family, learned to generate brain activity by attempting different movements. These brain signals are picked up by the implanted microelectrodes and are decoded by a machine learning model in real time. The model maps the signals to mean either ‘yes’ or ‘no’. To reveal what the participant wants to communicate, a speller program reads the letters of the alphabet aloud. Using auditory neurofeedback, the participant is able to choose ‘yes’ or ‘no’ to confirm or reject the letter, ultimately forming whole words and sentences.
The team is also working on ABILITY, a wireless implantable BCI device designed to flexibly connect to either microelectrode arrays or ECoG electrode grids. This will allow detection and processing of signals from either highly specific or larger areas of the brain. The approach could enable speech decoding directly from the brain during imagined speech leading to more natural communication.
The results show that communication is possible with people who are completely locked in because of ALS. The study was published in Nature Communications in March 2022.
Source: Wyss Center






